Application of hemoglobin oxygen carrier product HBOC-201 in the treatment of extensive burns
![]() 2024-08-01
2024-08-01
Treatment of burns is often accompanied by anemia and a high rate of allogeneic blood transfusion. In a study of patients with burns of 20% or more of their total surface area, Palmieri found that 75% received at least one unit of red blood cells during their hospital stay. This poses a great challenge to clinicians in their treatment. The mean total burn surface area of the deceased patients was 49.7% (range, 25-92.5%), whereas the mean burn extent of the survivors was 13.8% (range, 1-32.6%), and two of the survivors agreed to receive allogeneic blood transfusions. This article describes the use of HBOC-2011 in an elderly male burn patient who refused blood transfusion because of religious preference.
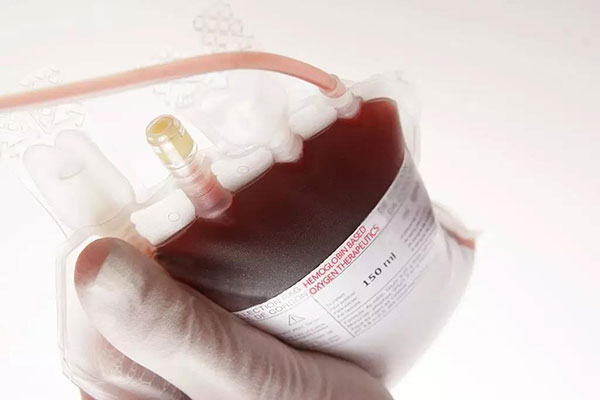
(1 Note: HBOC-201 is a bovine hemoglobin-based oxygen supply produced by Biopure, Cambridge, Mass.)
A 78-year-old man suffered burns after igniting an outdoor accelerant and was transferred to a burn center. He had burns covering approximately 42% of the body surface, most of which (39%) were full-thickness. Fiberoptic bronchoscopy confirmed the presence of respiratory tract injury. They agreed to the use of human albumin and hemostatic AIDS, including tranexamic acid and recombinant factor VII. Complementary measures to reduce the risk of anemia include:
(1) minimizing the frequency of blood sampling;
(2) use of pediatric blood transfusion tube;
(3) 40000 units recombinant human erythropoietin was used 3 times a week;
(4) Provide iron, folate, and vitamin B12.
At the end of the patient's early resuscitation, burn wound excision and wound closure were performed with minimal sequelae. Because of the size of the burn and the fact that the majority of burn wounds were on the trunk (which did not allow the use of hemostatic bands to reduce intraoperative bleeding), the judgment was that the patient would not survive the perioperative period without a blood supply. After discussion with the family, the hospital initiated the process of obtaining HBOC-201, which included the following steps:
(1) Clinicians with experience in using HBOC contact Biopure;
(2) Submit an application to FDA for approval of HBOC-201 as an emergency use Investigational New drug (E-IND);
(3) Submit a compassionate use protocol to a local institutional review board (IRB);
(4) Informed consent was obtained from the family members.
The application was approved on the basis of the potential for acute perioperative blood loss to cause a dramatic reduction in oxygen-carrying capacity and the potential for HBOC-201 to enhance survival.
The patient underwent burn excision, autograft, and allograft surgery with approximately 2500mL of blood loss without intraoperative infusion of HBOC-201. Approximately 4 hours after surgery, hypotension developed with a central venous oxygen saturation of 39% and a hemoglobin level of 5g per deciliter, and 4 units of HBOC-201 were infused over an 8-hour period. No adverse events occurred during the infusion of HBOC-201, and the patient remained normotensive. Ascorbic acid was also administered intravenously at a dose of 500 mg twice daily. Since HBOC-201 has a half-life of 19 hours, two additional units were infused the following evening. The patient's renal function also improved after infusion. Finally, the patient was successfully treated. He received a total of six units of HBOC-201 throughout.
HBOC-201 is designed to provide immediate oxygen delivery to hypoxic organs as a plasma expander and oxygen delivery agent in Settings where blood (red cells) is not available. Numerous studies have reported positive results using the original Biopure HBO-201 in animal models of trauma, brain injury, and blood loss. HBOC-201 has been approved for human use in South Africa and Russia. The FDA quickly approved the use of HBOC-201 for the treatment of burn patients who cannot receive allogeneic blood products. This case is one of many examples of compassionate use of this product in Europe and the United States in its early years.
-

Bios Wins the “Global Hemoglobin Oxygen Carrier Technology Innovation Leadership Award” at the Sullivan New Investment Conference 2025
On August 27, the 19th Sullivan Global Growth, Technology Innovation and Leadership Summit 2025 and the 4th New Investment Conference (hereinafter referred to as “Sullivan New Investment Conference 2025”) was grandly opened in Shangri-La Hotel, Jing ’an District, Shanghai.
2025-08-29
-

Wang Jianfeng, the Secretary of the CPC Changzhou Municipal Committee, met with Mr. Zheng Zhiheng, the Chairman of Bios Biologics
On 28 May 2025, Wang Jianfeng, Party Secretary of Changzhou City, Jiangsu Province, met with Mr. Zheng Zhiheng, Chairman of Bios Biotechnology Co., LTD., Vice Chairman and Executive Director of Chow Tai Fok Jewelry Group Co., LTD., to exchange views on further deepening cooperation.
2025-05-29
-
Yale scientists have successfully revived a pig brain with artificial blood
Recently, the cover of Nature published the latest research from Yale University School of Medicine: scientists at Yale University used an infusion solution to successfully revive the pig brain after 4 hours of death, restoring some cellular and metabolic functions, and maintaining them for at least 6 hours.
2024-08-03
-

A brief history of research and development of red blood cell substitutes
Blood has always been a very important, but very limited medical resource.
2024-08-02


