A brief history of research and development of red blood cell substitutes
![]() 2024-08-02
2024-08-02
Blood has always been a very important, but very limited medical resource. In order to develop clinically safe and effective blood substitutes, in the past half century, several generations of researchers around the world have launched a series of research on red blood cell substitutes, which can be called an epic history of founding.
The earliest red-cell substitutes were developed because of military needs and concerns about blood safety. To this day, the need for resuscitation fluid and blood substitutes in the armed forces is still in the balance, and other problems regarding routine transfusion have increasingly come up: blood shortage, blood cross-infection, transfusion ineffectiveness (e.g., AIHA), religious refusal to transfusion, and transfusion-related diseases (e.g., acute lung injury and venous thromboembolism). In this grim background, clinical first-line doctors and patients are calling for the development and marketing of red blood cell substitutes.

A blood substitute that can be used in clinical practice should have the following characteristics: no pathogens, no cross-matching, long validity, wide temperature storage, low immunogenicity, effectiveness is not lower than red blood cells, plasma volume expansion, rapid oxygen supply to tissues and organs, readily available, easy to implement.
Early attempts to test human-derived hemoglobin as a red-cell substitute in humans failed because of abdominal pain, a short intravascular half-life, and nephrotoxicity. Unmodified cell-free hemoglobin is ineligible for clinical use as a blood substitute because its oxygen affinity is too high to provide effective tissue oxygenation, its intravascular half-life is too short, and its propensity to dissociate in dimers, resulting in toxicity and kidney injury. In addition, because free hemoglobin tends to take up nitric oxide, this in turn causes vasoconstriction.
Since then, researchers have focused on two types of red blood cell substitutes, namely perfluorocarbon emulsions (PFCs) and Hemoglobin based Oxygen Carrier solutions (HBOC). Perftoran and Hemopure are the best of the two categories of red blood cell substitutes that have entered the clinic.

Fluorocarbon emulsions (PFCs)
PFCs are a class of chemically synthesized fluorocarbon compounds with high gas solubility, low viscosity, and physical and chemical inertness. Because they are difficult to dissolve in water, they need to be emulsified into soluble emulsions.
Fluosol-DA, a first-generation PFC, was approved by the FDA in 1989 for use in high-risk PTCA procedures because of its cardioprotective effect. Compared with its high efficacy of intracoronary-artery administration, it was ineffective in the treatment of anemia, and the oxygen it carried was released before it reached the capillary network.
The second-generation fluorocarbon emulsions were Oxygent (Alliance Pharmaceuticals) and Oxyfluor (HemoGen). The oxygen carrying capacity and stability of these products are improved compared with the first generation. The half-life of these products is 24 to 48 hours, and they can be stored at room temperature for more than 1 year without hemodynamic effects. Oxygent has completed phase III clinical trials in the United States, Europe, and Canada. However, it was found to increase the incidence of stroke in the late phase III clinical trial, so the clinical trial was suspended.
Perftoran, another product of fluorocarbon emulsions, has been successively approved for clinical use in Russia and Mexico. Perftoran infusion can promote oxygen delivery, alleviate ischemic symptoms of various obstructive vascular diseases, improve the survival of plastic surgery grafts, reduce inflammatory reactions, prevent graft rejection, activate liver detoxification function, inhibit retroviral infection, and accelerate wound and ulcer healing when applied locally, significantly reducing the need for re-transfusion of allogeneic red blood cells.
Several HBOCs have been developed over the past 30 years:
1.intramolecular cross-linked hemoglobin
Bis (3, 5-dibromosalicyl) fumarate was used by the United States Military and Baxter to cross-link the alpha subunits of human hemoglobin under deoxy conditions to produce succinic cross-bound hemoglobin (DCLHb, HemAssist). This product is relatively homogeneous and easy to control, while the oxygen affinity of the reaction product is improved. Thus, it was once favored by academia and business circles. But unfortunately, in the late phase Ⅲ clinical study, it was found that it did not show extraordinary effect in the rescue of hemorrhagic shock patients, and some patients died.
It was further found that the infusion of DCLHb could increase the mean arterial blood pressure, easily carry NO through the vascular wall, and cause vasoconstriction. As a result, Baxter halted its clinical trial in 1998. Human recombinant hemoglobin (rHbl.1 and rHb2.0) developed by Baxter Company showed beneficial biological effects in animal experiments such as the treatment of hemorrhagic shock, but they also have vasoconstrictive effects and often cause the increase of amylase and lipase, and further development of them was stopped in 2003.
2. Polyhemoglobin
Researchers first focus on chemical modification of hemoglobin molecules to stabilize its tetrameric structure and prevent its degradation to dimers and toxic side effects such as nephrotoxicity. Such products include Northfield's PolyHeme, Hemosol's Hemolink, and Biopure's Hemopure.
PolyHeme was developed as a military project after the Vietnam War. It is a glutaraldehyde polymerized human hemoglobin capable of increasing P50 partial pressure of oxygen at half saturation, with many advantages such as blood group restriction free, pathogen free, and 12-month storage period, and was developed as a replacement for stored red blood cells in surgery and trauma. Development of PolyHeme was discontinued in 2009 because of lack of FDA approval.
Hemosol, a Canadian company developing raffinose polymerized human hemoglobin (Hemolink), suspended its clinical trial of Hemolink after completion of clinical trials in cardiac surgery due to safety concerns (hypertension, jaundice, urine discoloration, and elevated pancreatic enzymes).
Hemopure (HBOC-201) is a chemically stable solution of bovine hemoglobin polymerized by glutaraldehyde. It has been licensed for human use in South Africa in 2001 and is the only red blood cell substitute approved for marketing in the world. "Unlike other products, Hemopure has satisfactory universal compatibility and stability when stored at room temperature (2-30 °C) for up to 3 years."
Its volume is 1/1000 of human red blood cells, and it is easy to pass through the obstruction of capillaries formed in patients with massive blood loss. It can quickly and efficiently replenish oxygen to the ischemic body, hypoxic tissues and organs or high-risk populations under the hypoxia environment at high altitude. Hemopure can significantly reduce the need for red blood cell unit transfusion in patients undergoing cardiac and abdominal aortic surgery, Hemopure is well tolerated as a resuscitation fluid during surgery, and clinical studies in orthopedic patients have shown broad prospects.
It is used in South Africa to reduce or delay the need for allogeneic red blood cell transfusions in patients presenting with symptoms of acute surgical anaemia. Due to the promising use of this product in animal studies, Biopure has also developed a similar product, called Oxypure, which has received marketing approval in the United States and Europe and has been widely used in veterinary clinics since March 1998. In 2002, the FDA accepted a Review application from Biopure to market the product for similar orthopedic indications. In the same year, the Naval Medical Research Institute was authorized to submit a new drug application to the US FDA.
The product has completed phase Ⅲ clinical trials in cardiac surgery, non-cardiac surgery and trauma. At the same time, another use of Hemopure, cardiac protection, has been explored in Europe, and has been confirmed in animal experiments. In a twist of fate, Biopure experienced a financial crisis in the US biopharmaceutical industry after its Nasdaq listing. In 2009, Biopure filed for bankruptcy protection and its assets were acquired by OPK Biotech.
However, the research and development of Hemopure is still not finished, OPK Biotech continues to work on the development of Hemopure for human use, and the US Navy continues to actively fund research with potential applications in war situations. Hemopure products were approved in Russia in 2010. The relay, which spans more than two decades, is still underway, and the dream is still going on.
In 2016, REDPHARM was established in Beijing. Redpharm announced the introduction of a technical team with Carl W. Rausch, the founder and chief scientist of Biopure, as the core, as well as clinical experts from Europe and the United States, and signed a full set of technology licensing agreements related to blood products.

3. Conjugated hemoglobin
Conjugation of hemoglobin with large inert molecules such as polyethylene glycol (PEG) can increase the molecular weight of hemoglobin, and the resulting conjugates can effectively prolong the circulating half-life of hemoglobin.
Enzon Corporation of the United States has developed PEG conjugated bovine hemoglobin. Succinimide-activated PEG was used to modify hemoglobin, which increased the molecular size, improved the surface properties of hemoglobin, reduced the immunogenicity, and obtained a longer circulation half-life in vivo. But Enzon was forced to halt the trial after reports suggested that PEG-conjugated hemoglobin could quickly leach out of the gastrointestinal mucosa and release blood-blocking substances from the intestinal mucosa into the bloodstream.
Scientists at Ajinomoto have developed a high-molecular-weight hemoglobin derivative with low oxygen affinity modified with pyridoxal phosphate and crosslinked with polyoxoethylene. The product was ultimately developed by Apex Biosciences, a subsidiary of Curacyte AG; It's called PHP. It is used as a nitric oxide scavenger in the treatment of systemic inflammatory response syndrome in stroke patients.
Phase I and II clinical trials have shown that PHP has the potential to restore normal hemodynamic effects and is well tolerated. No clinical side effects or laboratory abnormalities have been observed in excess of PHP. The phase III trial, published in 2014, failed due to increased mortality in the control group and led to the closure of Curacyte.
Hemospan is a pegylated hemoglobin developed by Sangart that is available in powder form. The product has a uniform modification degree, and the number of PEGs coupled to each hemoglobin molecule is similar, so the product uniformity is relatively good. It has a lower half-saturation partial pressure of oxygen P50, a larger molecular diameter, a higher viscosity, and no vasoconstrictor effect.
The higher oxygen affinity and the oxygen-releasing capacity of red blood cells and plasma hemoglobin in capillaries provide certain advantages in restoring circulation and tissue oxygenation. Although early clinical trials were promising, Sangart ultimately failed because it could not remain operational for financial reasons.
4. Hemoglobin microcapsules
Hemoglobin microcapsules mainly use expired blood samples to obtain purified hemoglobin by refining, and then use phospholipid bilayer membrane to enclose high concentration of hemoglobin molecules to form cellular particles. Due to the high cost of preparation and complex process, the United States gave up the development of this oxygen carrier, and now mainly by two Japanese universities and two companies to continue the research, in overcoming oxidation and optimization of oxygen affinity and other aspects have made significant progress.
The size of the hemoglobin microcapsules they produced by granulation technology was controlled to be about 250nm, so that there was no danger of oozing through the cracks in the blood vessel wall, and at the same time enough to allow oxygen transport through narrow capillaries.
5. Synthetic albumin-heme carrier
The synthetic albumin-heme carrier is a new type of fully synthetic artificial red blood cell, which uses recombinant albumin molecules synthesized by genetic recombination technology to carry artificial heme, and realizes oxygen transport function through its autonomous binding and dissociation of oxygen molecules. The albumin-heme carrier has good compatibility with human blood cells and does not cause vasoconstriction. It can be used not only as a red blood cell substitute, but also as an oxygen-carrying drug to effectively reoxygenate hypoxic solid tumors. However, there are still some problems that need to be further studied and solved before clinical application.
Each HBOC product has its own unique REDOX, chemical adhesive osmopressure properties, and intravascular half-life, which can affect specific clinical indication effects. "To date, however, only Biopure Hemopure has been approved for use as a red blood cell replacement in general surgical patients and for veterinary use in the United States and Europe (see table below)."
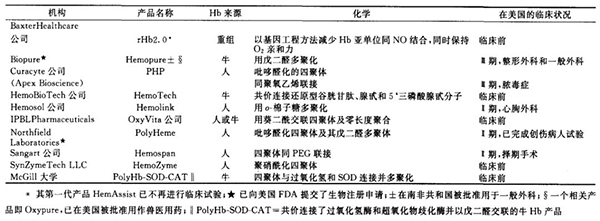
Epilogue:
The research of red blood cell substitutes (also known as "blood substitutes") has been a long-standing challenge for humans. Through more than half a century of unremitting efforts, this research has made remarkable progress. Each product of red blood cell substitutes has its own unique advantages and disadvantages. The main products of the first generation of red blood cell substitutes have entered phase Ⅲ or phase Ⅱ clinical trials, and some products such as Hemopure have even been used in clinical practice, which has made remarkable progress. After Runfang Bio takes over the baton of Hemopure, the product is expected to accelerate development and commercialization in recent years.

On the basis of the first generation of products, scientists tried a new generation of products, trying to make the red blood cell substitute can include the content of red blood cells (hemoglobin and various enzymes), so that it is closer to red blood cells. However, with the development of research and clinical trials, researchers found that it is difficult to make complete blood substitutes or red blood cell substitutes due to their extremely complex composition. It is difficult to really simulate blood, but it is of great practical significance to develop and research as an oxygen therapeutic agent for pre-hospital rescue of war injuries, accidental injuries, heart and stroke, transfusion related diseases, transfusion refusal due to religious reasons, and the treatment of endangered animals and service animals. It may become an important development direction for the development of red blood cell substitutes in the future.
-

Bios Wins the “Global Hemoglobin Oxygen Carrier Technology Innovation Leadership Award” at the Sullivan New Investment Conference 2025
On August 27, the 19th Sullivan Global Growth, Technology Innovation and Leadership Summit 2025 and the 4th New Investment Conference (hereinafter referred to as “Sullivan New Investment Conference 2025”) was grandly opened in Shangri-La Hotel, Jing ’an District, Shanghai.
2025-08-29
-

Wang Jianfeng, the Secretary of the CPC Changzhou Municipal Committee, met with Mr. Zheng Zhiheng, the Chairman of Bios Biologics
On 28 May 2025, Wang Jianfeng, Party Secretary of Changzhou City, Jiangsu Province, met with Mr. Zheng Zhiheng, Chairman of Bios Biotechnology Co., LTD., Vice Chairman and Executive Director of Chow Tai Fok Jewelry Group Co., LTD., to exchange views on further deepening cooperation.
2025-05-29
-
Yale scientists have successfully revived a pig brain with artificial blood
Recently, the cover of Nature published the latest research from Yale University School of Medicine: scientists at Yale University used an infusion solution to successfully revive the pig brain after 4 hours of death, restoring some cellular and metabolic functions, and maintaining them for at least 6 hours.
2024-08-03
-

A brief history of research and development of red blood cell substitutes
Blood has always been a very important, but very limited medical resource.
2024-08-02


